
Table of Contents
Introduction
Optical Emission Spectroscopy (OES) is an analytical technique used to determine the elemental composition of a sample by analysing the light emitted from atoms or ions excited to higher energy states. It has found widespread application in various fields, including metallurgy, environmental monitoring, quality control in manufacturing, and chemical analysis. OES is valued for its speed, accuracy, and versatility, allowing for the identification and quantification of a wide range of elements in different matrices, such as metals, liquids, and gases.
Principle of Operation
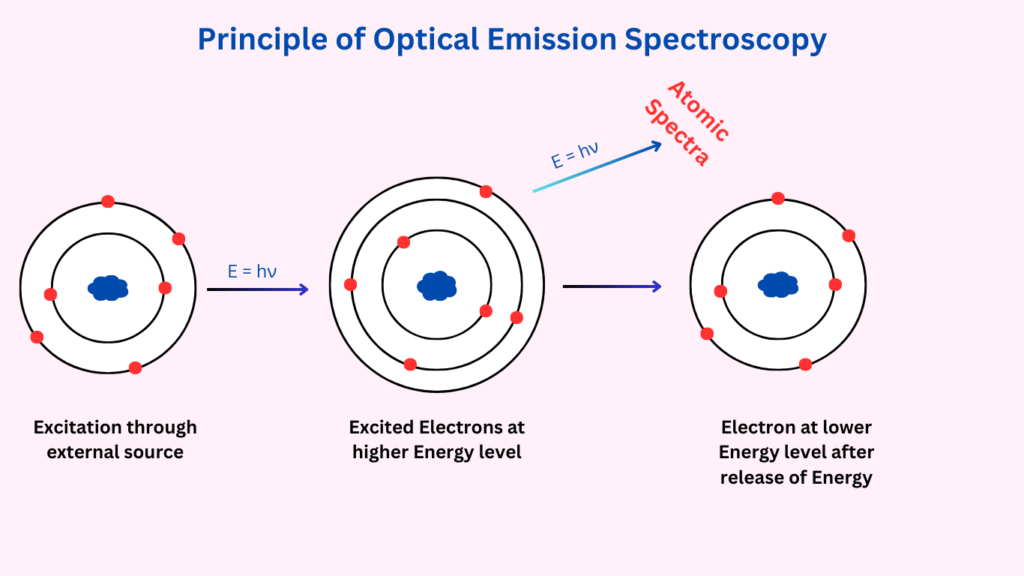
The fundamental principle of Optical Emission Spectroscopy revolves around the excitation of atoms in a sample, followed by the emission of photons as these atoms return to their ground state. When a material is exposed to a high-energy source, such as an electric arc, spark, or plasma, the atoms and ions in the material absorb energy and become excited. As they revert to their stable, lower energy states, they release energy in the form of light. The wavelength and intensity of the emitted light are characteristic of the specific elements present in the sample. The emitted light is then captured and analysed by a spectrometer, which disperses it into its component wavelengths. By measuring the intensity of these wavelengths, the concentration of each element can be determined.
Types of OES
There are several types of OES techniques, each distinguished by the method of excitation used to energize the atoms. The three main types include:
Arc/Spark Optical Emission Spectroscopy (Arc/Spark OES)
This method is widely used in the metals and alloy industry. In Arc/Spark OES, a high-voltage electrical discharge is used to excite the atoms in the solid sample. The discharge occurs between the sample and an electrode, producing a spark or an arc that vaporizes the sample’s surface. The resulting plasma contains excited atoms and ions that emit light, which is analysed by the spectrometer.
Arc/Spark OES is particularly well-suited for analysing metals and alloys, including steels, cast iron, aluminium alloys, and copper alloys. It provides precise measurements of the elemental composition of solid samples, making it ideal for quality control in the metallurgical industry.
Inductively Coupled Plasma Optical Emission Spectroscopy (ICP-OES)
In ICP-OES, a sample (typically a liquid) is introduced into a high-temperature plasma generated by a radiofrequency (RF) coil. The plasma is typically composed of argon gas, which serves as the medium for exciting the atoms and ions in the sample. As the sample is vaporized and ionized in the plasma, the resulting light emission is collected and analysed by the spectrometer.
ICP-OES is highly versatile and can analyse a broad range of elements in various matrices, including water, biological samples, and industrial effluents. It is widely used in environmental analysis, food safety, and pharmaceutical testing due to its high sensitivity and ability to handle multi-element analysis.
Glow Discharge Optical Emission Spectroscopy (GD-OES)
GD-OES employs a low-pressure glow discharge to excite atoms in the sample. The sample is placed in a vacuum chamber, and a low-pressure gas (usually argon) is introduced. A direct current (DC) or radiofrequency (RF) voltage is applied between the sample and the electrode, creating a plasma. The plasma etches the surface of the sample, and the emitted light from the excited atoms is collected and analysed.
GD-OES is ideal for depth profiling of materials, particularly thin films, coatings, and surface treatments. It is commonly used in material science and engineering to study the composition of layered structures and surface modifications.
Instrumentation
An OES system typically consists of the following main components:
Excitation Source
The excitation source provides the energy needed to excite the atoms or ions in the sample. Depending on the type of OES, the excitation source can be an electric arc, spark, plasma, or glow discharge. Each source has its strengths and is selected based on the sample type and the elements to be analysed.
Sample Introduction
In ICP-OES, the sample is typically introduced into the plasma as a liquid aerosol. In contrast, Arc/Spark OES and GD-OES involve direct solid samples, where the surface of the material is ablated or etched to produce a plasma for analysis.
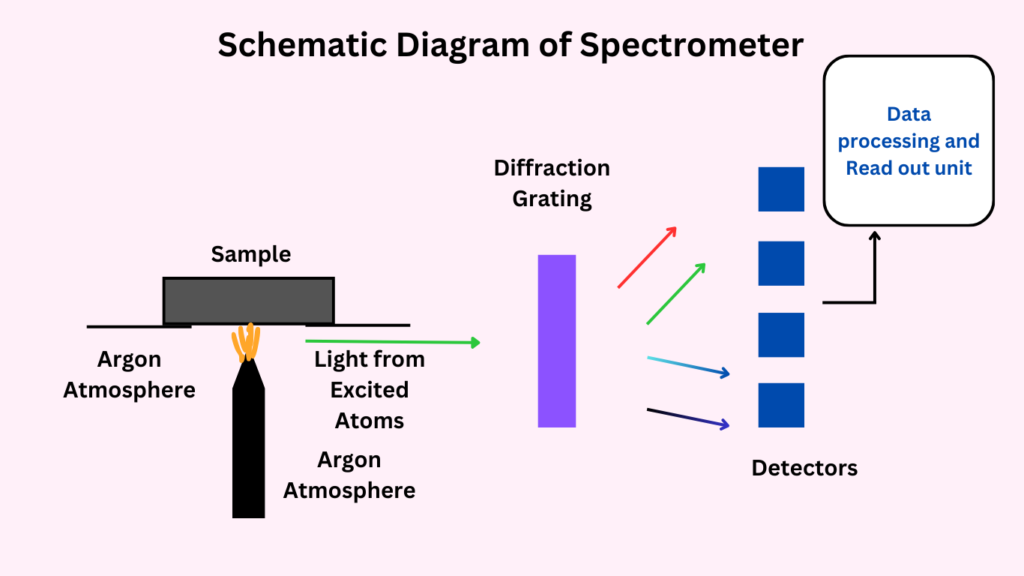
Optical System
The optical system includes a spectrometer, which disperses the emitted light into its component wavelengths. The spectrometer typically consists of a diffraction grating that separates light based on its wavelength. Detectors, such as charge-coupled devices (CCDs) or photomultiplier tubes (PMTs), measure the intensity of the light at each wavelength, allowing for the identification and quantification of elements.
Detection System
The detection system records the intensity of the emitted light at specific wavelengths, which is directly proportional to the concentration of the corresponding element in the sample. Modern OES systems are equipped with highly sensitive detectors that can capture a wide spectral range, ensuring the detection of multiple elements in a single analysis.
Data Processing
The collected data is processed using specialized software that compares the measured intensities with calibration data to quantify the elemental concentrations. Calibration standards are typically used to ensure the accuracy and precision of the results.
Advantages of OES
Optical Emission Spectroscopy offers several advantages, making it a preferred analytical technique in many industries:
Rapid Analysis
OES provides real-time, fast analysis, often delivering results within minutes. This is crucial in industries such as metallurgy and manufacturing, where time-sensitive quality control decisions must be made.
Multi-element Detection
OES can simultaneously detect and quantify multiple elements in a single analysis. This is particularly beneficial when analysing complex matrices or alloys containing several different elements.
High Sensitivity and Precision
OES offers excellent sensitivity, particularly in the case of ICP-OES, which can detect trace levels of elements down to parts per billion (ppb) or even lower. It also provides high precision, ensuring reliable and reproducible results.
Versatility
OES is applicable to a wide range of sample types, including metals, liquids, gases, and solid surfaces. Its versatility makes it suitable for use in numerous industries, from environmental monitoring to aerospace and defence.
Limitations and Challenges
Despite its many advantages, Optical Emission Spectroscopy also has several limitations and challenges:
Matrix Effects
The presence of complex sample matrices can interfere with the accuracy of the results. For example, in ICP-OES, high salt concentrations or organic compounds in the sample can affect the plasma’s stability and lead to inaccurate readings. Careful calibration and sample preparation are necessary to mitigate these effects.
Limited Sensitivity for Some Elements
While OES is highly sensitive for many elements, it may not provide sufficient sensitivity for certain elements, particularly non-metals like hydrogen, oxygen, and nitrogen. Specialized techniques, such as elemental analysers or mass spectrometry, may be needed to complement OES in such cases.
Instrument Cost and Maintenance
OES systems, especially ICP-OES and GD-OES, can be expensive to purchase and maintain. The instruments require regular calibration, cleaning, and maintenance to ensure accurate and reliable performance. The high initial cost and operational expenses can be prohibitive for smaller laboratories.
Sample Preparation
Some OES techniques require extensive sample preparation, especially for solid samples. In Arc/Spark OES, for instance, the surface of the metal sample must be carefully cleaned and polished to avoid contamination, which can affect the accuracy of the analysis.
Applications of Optical Emission Spectroscopy
Optical Emission Spectroscopy is employed in a wide range of industries and applications due to its versatility, speed, and accuracy:
Metallurgy and Materials Science
OES is extensively used in the analysis of metals and alloys, particularly in quality control during production processes. It helps ensure that materials meet the required specifications for elements such as carbon, silicon, and sulphur in steel production, or aluminium, magnesium, and zinc in aluminium alloys.

Environmental Monitoring
ICP-OES is widely used in environmental analysis to detect trace elements in water, air, and soil samples. It plays a crucial role in monitoring pollutants such as heavy metals (e.g., lead, mercury, arsenic) in drinking water, industrial effluents, and atmospheric emissions.
Pharmaceutical and Food Industries
OES is applied in the pharmaceutical and food industries for trace element analysis. For example, ICP-OES is used to ensure the safety and quality of pharmaceutical products by detecting trace levels of contaminants or residual elements. In the food industry, it is employed to analyses nutritional content and detect harmful elements like cadmium or lead.
Aerospace and Defence
OES plays a critical role in the aerospace and defence industries, where the precise composition of metals and alloys used in aircraft and military equipment is vital for safety and performance. Arc/Spark OES is often used to analyse the composition of turbine blades, engine parts, and structural components.
Energy and Power Generation
In the energy sector, OES is used to analyse materials used in power generation, including turbines, boilers, and nuclear reactors. Accurate elemental analysis ensures the structural integrity and performance of these critical components.
Conclusion
Optical Emission Spectroscopy (OES) is a powerful, versatile analytical technique that offers rapid, accurate, and multi-elemental analysis across a wide range of materials and industries. Its ability to detect and quantify elements with high sensitivity and precision makes it indispensable in fields such as metallurgy, environmental monitoring, pharmaceuticals, and aerospace. While there are challenges, such as matrix effects, cost, and sample preparation requirements, the benefits of OES—including its speed, non-destructive nature (where cutting and shaping are not required), and adaptability—outweigh these limitations for many applications. As technological advancements continue to refine instrumentation and data processing, OES is poised to become even more efficient and accessible. Its role in quality control, safety assurance, and environmental protection will remain critical in ensuring the reliability and sustainability of processes and products in the modern world.

